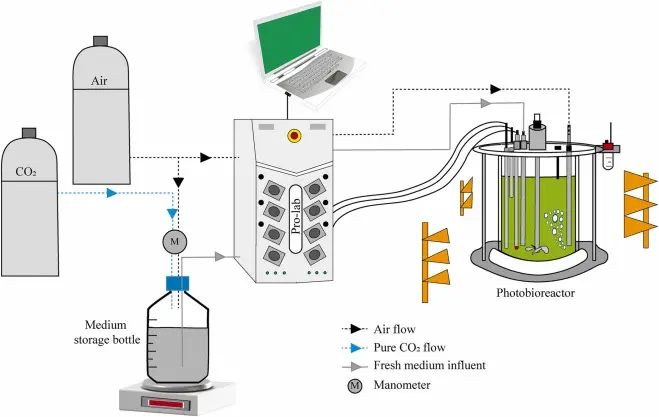
Biologic Process Development
"From gene to production : trusted lab services in molecular biology and bioprocessing"
About Us
From gene to protein, from cell to solution , we provide the tools, technology, and expertise to bring your life science projects to life. Partner with us to streamline research, optimize processes, and scale up with confidence.



Where Biotech Ideas Become Breakthroughs
From gene to protein, from cell to solution , we provide the tools, technology, and expertise to bring your life science projects to life. Partner with us to streamline research, optimize processes, and scale up with confidence.
Get More Informations
Our Mission
This organisation operates as an outsourced contract laboratory, meaning it provides specialised scientific services to other companies, universities, or research institutions that prefer not to build or maintain their own facilities for these tasks. Here’s a breakdown of what they offer.
Contact-Us
What Is Biologic Process Development?
Biologic process development is the foundation of modern biotechnology, guiding the transformation of living cells into life-saving therapies, vaccines, and biomolecules. Whether you’re producing monoclonal antibodies, recombinant proteins, or gene therapy vectors, this process bridges the gap between scientific discovery and scalable, clinical-grade manufacturing. In this guide, we break down the key steps from upstream cell culture to downstream purification so newcomers can understand how biologics are brought to life.

Upstream to Innovation: Mapping the Modern Biologic Proces
Biologic process development includes key stages such as cell line development, media optimisation, and bioreactor strategies like batch, fed-batch, and perfusion to grow cells and produce biologics. This is followed by purification and formulation to ensure product quality and safety. Understanding the distinction between upstream and downstream processes is essential. To improve efficiency and better mimic real tissues, innovative techniques like 3D cell culture are now gaining traction, transforming how biologics are developed and tested.

Batch Culture: Simplicity with Predictability
Batch culture is the most straightforward approach to bioproduction, where cells are grown in a fixed volume of nutrient-rich media without any further additions during the cultivation process. This closed system offers simplicity, ease of setup, and reproducibility, making it ideal for early-stage development and small-scale runs. However, its limitations lie in the finite availability of nutrients and accumulation of waste, which can restrict cell growth and productivity. Today, batch systems are being enhanced with smart sensors and inline analytics that monitor culture conditions in real time, allowing operators to better predict peak performance windows and automate harvest timing making even simple batch systems more intelligent and efficient.
Fed-Batch Culture: Adaptive Growth for Enhanced Yields
Fed-batch culture builds upon the batch model by allowing controlled addition of nutrients throughout the growth phase, extending cell viability and boosting protein expression. This technique has become the gold standard in industrial biologics production due to its flexibility and higher yields. With the integration of AI-guided feeding strategies, metabolite monitoring, and predictive modelling, fed-batch systems are now more adaptive than ever responding dynamically to the metabolic needs of the culture. This level of precision not only improves product consistency but also reduces resource waste and production costs, positioning fed-batch as a key driver in the evolution of smart, scalable bioprocessing.
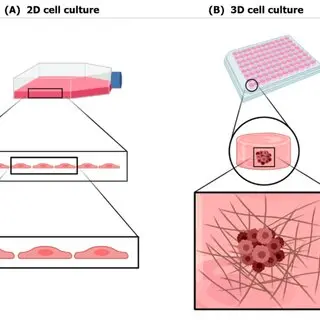
2D vs 3D Cell Culture : Why the Future of Bioprocessing Is 3D
Traditional 2D cell culture systems, where cells grow in flat monolayers on plastic surfaces, have been the cornerstone of biological research and bioprocess development for decades. However, these models often fail to replicate the complex architecture and microenvironment of living tissues, leading to limitations in predicting how cells behave in vivo. In contrast, 3D cell culture enables cells to grow in all directions, forming structures that more closely mimic human tissues in terms of morphology, gene expression, and function. This spatial complexity allows for more accurate modeling of disease states, drug responses, and tissue-specific interactions—key advantages in biologic development. From organoids and spheroids to scaffold-based and hydrogel systems, 3D culture methods are being integrated into upstream processes to improve productivity, cell viability, and translational relevance. As bioprocessing shifts toward precision medicine and complex biologics, 3D cell culture stands out as a transformative tool, enhancing not only preclinical modelling but also biomanufacturing strategies, paving the way for more predictive, efficient, and scalable biologic development.
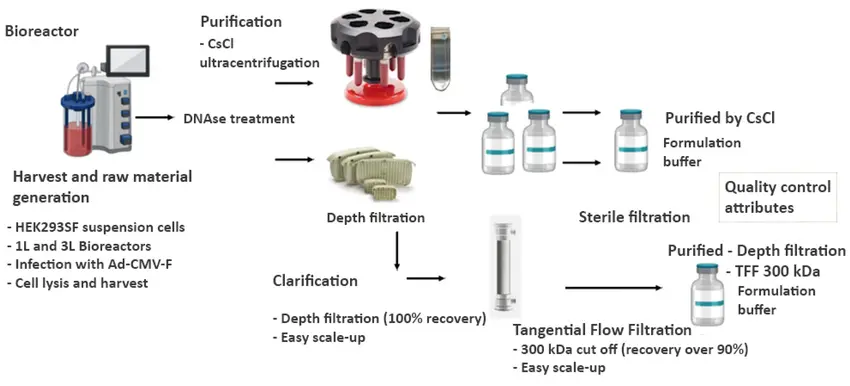
The Science Behind Biologics: Purification and Formulation
Following upstream production, biologic therapies undergo purification and formulation to ensure product quality, safety, and stability. Purification involves isolating the target moleculesuch as a monoclonal antibody or recombinant protein from a complex mixture of host cell proteins, nucleic acids, and process-related impurities. This is typically achieved through a series of steps including centrifugation, filtration, and chromatography techniques like affinity, ion exchange, and size exclusion, which together help achieve high purity without compromising biological activity. Once purified, the biologic is subjected to formulation, where it is combined with buffers, stabilizers, and excipients that enhance solubility, protect against degradation, and ensure a suitable shelf life. Formulation also accounts for dosage form and delivery method, whether as a liquid solution, lyophilized powder, or injectable product. Together, purification and formulation bridge the gap between raw biologic production and the creation of a stable, safe, and patient-ready therapeutic.
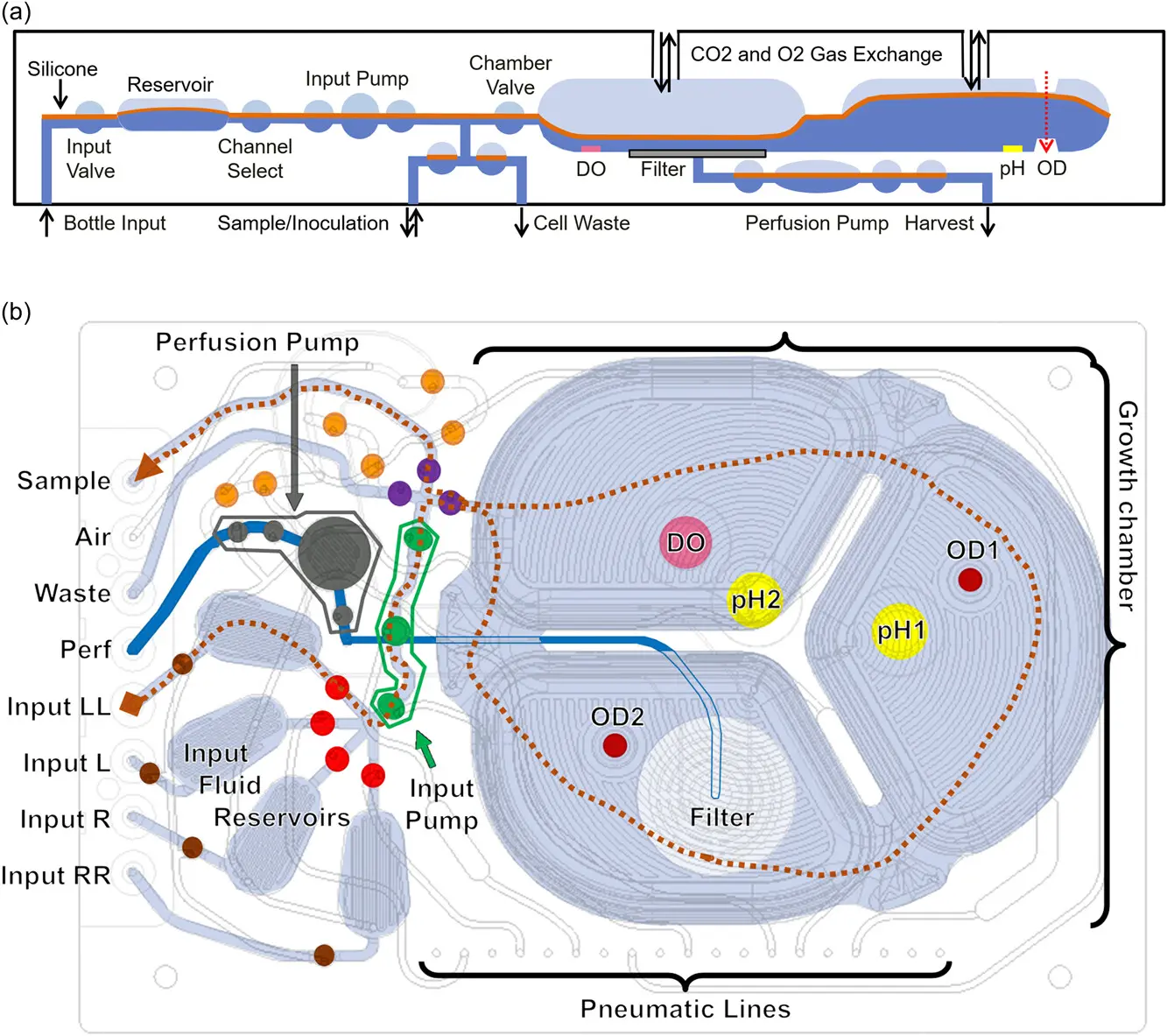
High-Density Cell Culture with Perfusion: A Guide for Bioprocess Engineers
Perfusion culture is an advanced bioprocessing strategy in which fresh nutrient-rich media is continuously supplied to the bioreactor, while spent media containing waste products is simultaneously removed. This constant renewal maintains cells in their optimal growth phase for extended periods, preventing nutrient depletion and toxic byproduct accumulation. As a result, perfusion enables exceptionally high cell densities and sustained biologic production, making it ideal for producing complex or fragile therapeutic proteins that require long culture durations. Unlike batch and fed-batch systems, perfusion demands sophisticated control systems to monitor flow rates, cell viability, and nutrient balance in real time. These systems often integrate automated sensors, feedback loops, and cell retention devices such as hollow fiber filters or spin filters to keep cells in the bioreactor while allowing waste to exit. While more technically demanding and resource-intensive, perfusion is gaining momentum in modern biomanufacturing due to its efficiency, scalability, and suitability for continuous processing platforms.
Alternating Tangential Flow (ATF) Filtration: A Game-Changer in Cell Retention
ATF filtration is a leading cell retention technology designed to support high-density perfusion cultures by efficiently separating cells from spent media without disrupting cell viability. The system operates by creating a gentle, back-and-forth (alternating) flow across a hollow fiber membrane using a diaphragm pump. This alternating motion minimizes membrane fouling and shear stress, allowing for longer run times and more stable performance compared to traditional filtration methods. Because cells remain in the bioreactor while waste and product flow through the filter, ATF enables continuous operation at very high cell concentrations—sometimes exceeding 100 million cells per milliliter. Its closed, automated design also reduces contamination risks and supports GMP compliance, making it ideal for clinical and commercial-scale manufacturing of biologics. Additionally, ATF systems are scalable, available in various sizes, and compatible with both microbial and mammalian cell cultures. As perfusion-based processes gain traction in the industry, ATF has emerged as a critical enabler of efficient, robust, and scalable cell culture systems.